Traditional treatment options for DPN have focused on blood sugar management and symptomatic relief, but emerging therapies like Spinal Cord Stimulation (SCS) are showing great promise.
What is Spinal Cord Stimulation (SCS)?
Spinal cord stimulation is a minimally invasive therapy that uses an implantable device to deliver mild electrical pulses to the spinal cord. These electrical impulses interfere with pain signals being sent to the brain, providing relief for chronic pain conditions.
A spinal cord stimulator (SCS) device is placed under your skin and sends a mild electric current to your spinal cord. Thin wires carry current from a pulse generator to the nerve fibers of the spinal cord. When turned on, the SCS stimulates the nerves in the area where your pain is felt. Pain is reduced because the electrical pulses modify and mask the pain signal from reaching your brain.
Who is a candidate?
An evaluation of your physical condition, medication regime, and pain history will determine whether your goals of pain management are appropriate for SCS. A neurosurgeon, physiatrist, or pain specialist will review all previous treatments and surgeries. Because chronic pain also has emotional effects, a psychologist will assess your condition to maximize the probability of a successful outcome.
Patients selected for SCS usually have had chronic debilitating pain for more than 3 months in the lower back, leg (sciatica), or arm. They also typically have had one or more spinal surgeries.
You may be a candidate for SCS if :
- Conservative therapies have failed.
- You would not benefit from additional surgery.
- The pain is caused by a correctable problem and should be fixed.
- You do not want further surgery because of the risks or long recovery. Sometimes SCS may be chosen over a large, complex spine surgery.
- You do not have untreated depression or drug addiction; these should be treated prior to having a SCS.
- You have no medical conditions that would keep you from undergoing implantation.
- You have had a successful SCS trial.
SCS has been used successfully for treating chronic pain conditions, such as failed back surgery syndrome and complex regional pain syndrome, but now, evidence has proven that it is an effective treatment for diabetic peripheral neuropathy.
What happens during spinal cord stimulation?
Spinal cord stimulator implantation is a process that usually involves two procedures. The first is a “trial” procedure. The second — if the trial succeeds — will involve the full surgery to implant the pulse generator or battery. (The preparations before both procedures, as noted in the above, “What happens before this procedure?” section, are the same.)
A spinal cord stimulator involves attaching one or more electrical leads (insulated wires or small, flat panels with conductive contacts near the tip). The conductive parts of leads go into the epidural space, which is between the dura mater (the outer membrane that surrounds the spinal cord itself) and the ligaments on the inside of your spine. The leads connect to a pulse generator, which generates the electrical current that will stimulate the spinal cord.
The location of the leads on your spinal cord can vary. For most people, the best place is in your back at about the same level as the lower edge of your breastbone (sternum). For those with pain in the arm, the neck may be a possible option for placement.
Trial procedure
The trial procedure generally involves a percutaneous (meaning “through the skin”) approach. During this procedure, you’re usually under sedation (light sleep). You might receive general anesthesia under certain circumstances, but this is less common. They’ll also use a special type of x-ray called fluoroscopy (the real-time video equivalent of an x-ray), which lets them see where to place the lead.
After you’re under sedation or anesthesia, your provider makes a small incision (cut) in your skin and inserts a special needle through the incision and into your back. Once the needle tip reaches epidural space, your provider will thread the temporary lead(s) into position and remove the needle, leaving part of the lead outside your skin.
Once the leads are in place, your provider will secure the external end of the lead to your skin and connect it to a pulse generator, also attached to your skin (attaching the lead and the external generator can use a suture or skin glue). They’ll then program and start testing to see whether or not the stimulator works without having to do a full surgery.
These trials typically last between a few days to a few weeks (this varies in different parts of the world). Your provider will remove these leads at the end of the trial. Experts consider a stimulator trial successful if you have at least a 50% decrease in pain level. If the trial is successful, most people undergo the next step, implantation surgery, about a week or two after the trial procedure.
If a percutaneous electrode isn’t possible, your provider may recommend placing a paddle electrode instead. This is a wider electrode that requires additional surgery to make room for it. If you have a paddle electrode implanted, you’ll need to stay overnight in the hospital.
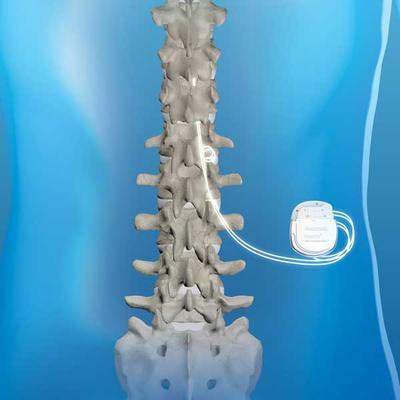
SCS Implantation surgery
During this procedure, which involves general anesthesia, your provider will perform surgery to place permanent leads in the same location as the trial. That starts with an incision (cut) over your spine. If your provider is placing a paddle electrode, they’ll need to make a space to pass through muscle tissue for the electrode to fit through. If they’re placing a percutaneous electrode, they won’t need to create that kind of space.
Once they place the leads, they’ll anchor the electrode end of the lead(s) into place and thread the near end of the lead under your skin to where they’ll implant the pulse generator. They’ll then create a small, pocket-like space where they can implant the pulse generator.
Where they implant the pulse generator depends on a few factors. They’ll usually place the generator just above or below belt level on the side that’s easiest for you to reach (either on the belly side or in one of your buttocks). They may also put it on the opposite side of whichever side is your preference for sleeping.
Being able to reach the skin over the top of the pulse generator is important, depending on which type of generator you have implanted. Some pulse generators are constantly active. Others need switching off and on, which you do with a remote you hold next to the generator. There are also rechargeable generators, which you charge by holding a special charging device above or against the skin just over the generator.
Once your provider finishes implanting the generator, they’ll use sutures and/or staples to close the incisions. The process of programming the generator can happen in the operating room, the recovery room after surgery or both. They can also make adjustments to the programming at follow-up appointments.
What happens after this procedure?
After the trial procedure, you’ll likely be able to go home that same day or the following day. After the generator implantation surgery, most people can go home within one or two days.
Your healthcare provider will give you guidance and instructions on caring for the procedure site after the trial lead placement and the generator implantation. In most cases, that involves taking care of the surgical wound, keeping it clean and changing its bandages for seven to 10 days. You’ll have a follow-up appointment about 10 to 14 days after the generator implantation surgery, where your provider will remove the staples and/or stitches.
Conclusion
Spinal cord stimulation is an emerging treatment option for diabetic peripheral neuropathy, and current research suggests that it can be a safe and effective method for managing pain associated with this condition.
The studies mentioned in this post provide compelling evidence of the benefits of SCS, such as significant pain relief, improved quality of life, and reduced medication use.
If you’re suffering from diabetic peripheral neuropathy and are interested in SCS as a potential treatment option, contact our office today for consultation with one of our expert physicians!